Our portfolio
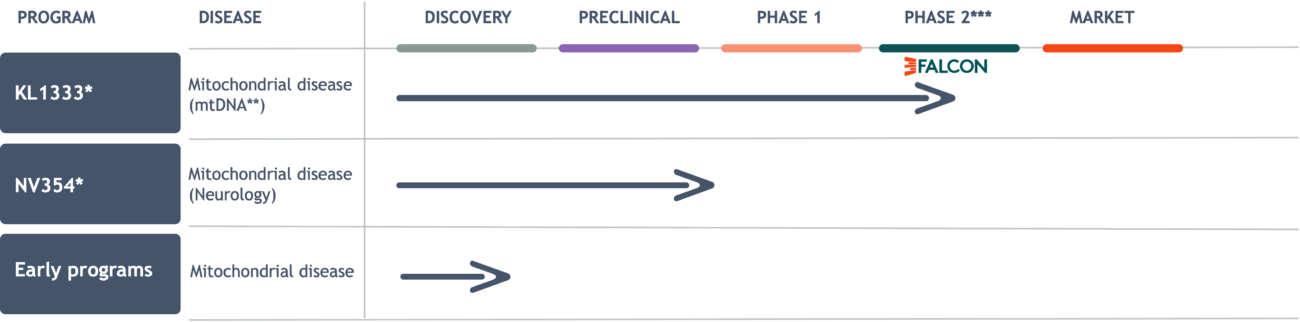
**mtDNA-related mitochondrial disorders caused by mutation(s) in mitochondrial DNA (as opposed to nuclear DNA).
***Given that mitochondrial diseases are orphan diseases, a Phase 2 study in these patients, if successful, has the potential to be considered registrational.
KL1333: Innovative therapy in late-stage development
Project status: Positive interim analysis of data from patients in Wave 1 of the FALCON study
FALCON is a Phase 2, global, randomized, placebo-controlled, potentially registrational study evaluating the safety and efficacy of KL1333 in adult patients with primary mitochondrial disease who experience consistent, debilitating fatigue and myopathy (muscle weakness), the most common and impairing symptoms.
A total of 180 patients with mitochondrial DNA mutations who meet the eligibility criteria are randomized 3:2 to receive KL1333 (50mg-100mg) or placebo twice daily for 48 weeks. The two alternative primary endpoints assess consistent fatigue (using the PROMIS Fatigue Mitochondrial Disease Short Form) and myopathy (using the 30 second Sit-to-Stand test), only one of which must be positive to file for marketing approval.
In June 2023, the first patient was dosed. In July 2024, an interim analysis evaluating 24-week data from the first wave of patients confirmed the strong safety profile of KL1333, and both primary endpoints passed futility, meaning that both have the potential to demonstrate benefit in the final analysis of the study.
KL1333 has Orphan Drug Designation (ODD) in both Europe and the U.S. as well as Fast Track designation in the U.S.
Mitochondrial disease target population: MELAS-MIDD, KSS-CPEO and MERRF
Abliva’s lead candidate, KL1333, has been designed to treat consistent fatigue and myopathy (muscle weakness) in genetically confirmed adult patients with primary mitochondrial disease. Diagnoses can include MELAS-MIDD and KSS-CPEO spectrum disorders as well as MERRF syndrome. The drug candidate is intended for long-term oral treatment.
Mechanism of action: NAD⁺/NADH modulation
KL1333 is a potent modulator of the cellular levels of NAD⁺ and NADH, central co-enzymes in the cell’s energy metabolism. This modulation leads to the formation of new mitochondria and improved energy levels at the cellular level which is expected to translate into a system-wide improvement in energy and organ function of patients in the FALCON study.
NV354: First-in-class therapeutic targeting high unmet need
Project status: Preclinical development completed
The program has completed preclinical development and has been granted Orphan (Drug) Designation both in the EU and the U.S. Given the prioritization of KL1333, no cost-intensive operational activities are planned for NV354 at this time.
Mitochondrial disease target population: Neurology
NV354 is being developed for mitochondrial disease with neurologic complications, in particular at insufficient activity in the mitochondrial protein complex I. The resulting deficiency in energy conversion contributes to clinical signs and symptoms in many types of mitochondrial disease, including neurologic complications seen in Leigh syndrome, MELAS, or LHON. There are also additional expansion opportunities outside of mitochondrial disease, including neurologic conditions where mitochondrial dysfunction has been confirmed.
The drug candidate was discovered due to its ability to increase mitochondrial function in cells from mitochondrial Leigh syndrome patients. Leigh syndrome usually debuts at one to two years of age and includes psychomotor regression, hypotonia and developmental delays. The disease is fatal, and children with early-onset Leigh syndrome usually die before adulthood.
Mechanism of action: Energy replacement
Brain-penetrable NV354 is based on an innovation in which the body’s own energy substrate, succinate, is made available in the cell via a prodrug technology. A prodrug is an inactive drug that is activated first when it enters the body by the transformation of its chemical structure.
Early programs
Abliva has efforts ongoing to identify additional portfolio opportunities focused on the regulation and stabilization of cellular energy production.