In simplified terms, the mitochondria can be considered as the energy factory of cells. There is currently scientific data indicating that many medical conditions, from chronic diseases to acute conditions affecting the heart and brain, are linked to their function. Succinic acid is a central component in energy production. This compound is involved in many pathogenic changes to energy production, which means that adding succinic acid can restore energy production. However, succinic acid is normally unable to transit the cell wall independently. NeuroVive’s and Selcia’s researchers have resolved this problem by altering succinic acid in various ways, with the result that the compound is able to transit the cell wall and then be freed up and utilized in mitochondrial energy production.
Therapy areas and current development work
The new drug candidates may potentially be used to prevent or treat primary or secondary mitochondrial conditions and restore normal function. This means that if current development work is successful, they may be suitable to treat a large number of rare genetic conditions, as well as more general disorders where there is an increased need for energy. This dual application extends the potential for this new class of pharmaceutical.
These new experimental drugs are currently undergoing formulation work. Their efficacy and potential adverse events must be examined in animal studies before they can be trialed on humans. The first major validation study on animals will be conducted in 2014.
New medical analysis method for mitochondrial function in human cells
NeuroVive’s research team has also developed a medical analysis method for energy regulation in human cells that enables the study of molecules and pharmaceuticals that can directly increase or decrease energy production. This method, called ToxPhos®, has the potential for future use to support the diagnosis of mitochondrial disease and medical conditions. The method, although not used to produce the ‘Amber’ compounds, was central in the screening process of the energy-regulating experimental drugs and is the foundation for the development of several preclinical models for mitochondrial conditions. The patent application for this method was also published in the WIPO patent database on 10 April.
”Research into mitochondrial medicine in recent years has demonstrated that far more diseases and medical conditions may potentially be sourced from mitochondrial defects than previously believed. That’s why it was a natural move for us to start developing pharmaceuticals that not only protect the mitochondria in cell damage, but also offer the potential for cells to produce normal amounts of energy in injury and disease. These new experimental drugs that we’re developing may potentially be used as emergency treatment in cellular energy crisis in children with congenital limitations to energy production and, potentially, for other conditions where raised energy production counters the course of diseases. We’re really proud to be able to present the underlying scientific discoveries at this symposium in June,” commented Eskil Elmér, NeuroVive’s CSO.
“The new experimental drugs have the potential to address a market worth billions of kronor and a substantial medical need in the treatment of rare diseases that affect the mitochondria. This application has great potential to secure orphan drug designation, which means a fairly speedy process prior to market launch and a long period of market exclusivity. These diseases usually affect children, which makes it extra urgent to start designing a suitable formulation and begin preparation of preclinical safety testing required before experimental drugs can be administered to patients in clinical trials,” continued Mikael Brönnegård, NeuroVive’s CEO.
Presentation at an international symposium in June
These new experimental drugs and the underlying scientific discoveries, including the new medical analysis method ToxPhos®, will be presented by NeuroVive’s researchers at the United Mitochondrial Disease Foundation Symposium in Pittsburgh on June 4-7. There’s more information on the conference at www.umdf.org.
About NeuroVive
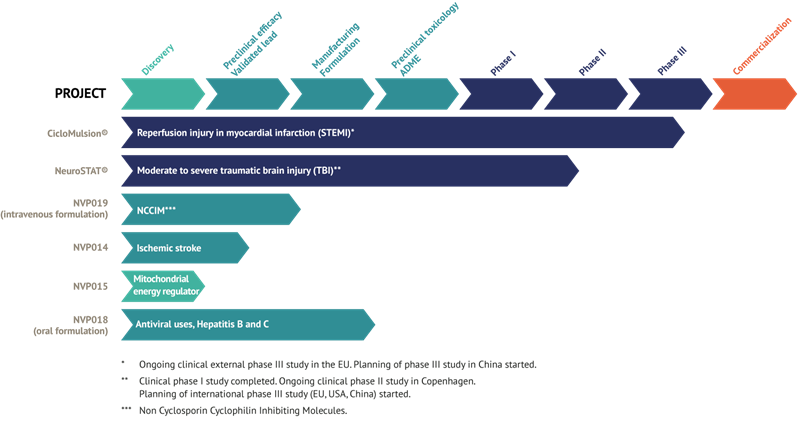
NeuroVive Pharmaceutical AB (publ), a leading mitochondrial medicine company, is developing a portfolio of products to treat acute cardiovascular and neurological conditions through mitochondrial protection. These medical conditions are characterized by a pressing medical need and have no approved pharmaceutical treatment options at present. NeuroVive’s products CicloMulsion® (heart attack) and NeuroSTAT® (traumatic brain injury) are currently being evaluated in phase III and phase II studies, respectively. NeuroVive’s research programs also include products for the treatment of anti-viral indications (Hepatitis B/C), brain cell injury in stroke patients, and drug candidates for cellular protection and treating mitochondria-related energy regulation diseases. NeuroVive’s shares are listed on NASDAQ OMX, Stockholm, Sweden.
Current status of NeuroVive’s products
CicloMulsion®
NeuroVive’s product CicloMulsion® is the first cyclophilin inhibitor developed for treating reperfusion injury. The product’s potential for treatment of myocardial infarction is currently being evaluated in a clinical phase III study. The final participant of a total of 972 patients was enrolled on 16 February 2014. The results of this study are scheduled for presentation in 2015 after a one-year follow-up is completed on all patients and study data has been compiled.
NeuroSTAT®
NeuroVive is developing NeuroSTAT® for treating patients with severe traumatic brain injury (TBI). NeuroSTAT® is currently being evaluated in a clinical phase IIa study at Rigshospitalet, Copenhagen. The study is focusing on safety and pharmacokinetics, and 5 of a planned 20 patients have been enrolled. The design and planning work for a phase III study has commenced. The company has obtained orphan drug designation for NeuroSTAT® for moderate to severe cranial injury in the US and EU. Orphan drug designation confers market exclusivity for 7 years in the US and 10 years in the EU from the date when the company secures marketing authorization.
NVP018
The recently acquired cyclophilin inhibitors are part of a family of molecules called Sangamides, and based on a new and unique chemical platform of what are known as polyketides. NVP018 is NeuroVive’s primary drug candidate in the company’s new portfolio of potent cyclophilin inhibitors. It has undergone extensive preclinical development and has been developed for treating hepatitis B/C. This product demonstrates potent efficacy against virus replication and has a positive safety and pharmacokinetic profile. Cyclophilin inhibitors have the potential for broad-based application, and NeuroVive is also currently evaluating NVP018’s potential against other viral indications.
Other products
More information on all products developed by NeuroVive is available at www.neurovive.se/index.php/en/research-development/our-products
For media and investor relations questions, please contact:
Ingmar Rentzhog, Laika Consulting, tel: +46 (0)46 275 62 21 or ir@neurovive.se
It is also possible to arrange an interview with NeuroVive’s CEO Mikael Brönnegård via the contact above.
NeuroVive Pharmaceutical AB (publ)
Medicon Village, SE-223 81 Lund, Sweden
Tel: +46 (0)46 275 62 20 (switchboard), Fax: + 46 (0)46 888 83 48, info@neurovive.se, www.neurovive.se
NeuroVive Pharmaceutical AB (publ) is required to publish the information in this news release under The Swedish Securities Market Act. The information was submitted for publication on 17 April 2014, at 8:45 a.m. CET.