The results indicate that NVP018 has a dual effect against the hepatitis B virus. Firstly, the data generated suggests that NVP018 directly inhibits several stages in viral propagation in liver cells, and secondly, NVP018 also operates indirectly by strengthening the immune response. The data also indicates that the risk of developing resistance, a significant clinical problem with current therapy alternatives for hepatitis B, is very low with NVP018. Finally, NVP018 also demonstrates activity in an animal model of chronic hepatitis B infection.
According to the WHO, over 2 billion people have been in contact with the hepatitis B virus and 240 million people have developed chronic hepatitis B. This means that the disease is one of the largest global medical challenges. The chronic disease is estimated to cause more than 600,000 deaths yearly through advanced cirrhosis of the liver or primary liver cancer. The disease is worldwide, but most common in Southeast Asia, Eastern Europe and Africa, with over 8% chronic carriers. In Western Europe and the US, the frequency is less than 1%. Currently available pharmaceuticals display limited effectiveness and there is a significant risk of the virus developing resistance, so accordingly, there is a substantial need for new pharmaceuticals.
NVP018 is an oral preparation of NeuroVive’s leading drug candidate in the company’s new portfolio of cyclophilin inhibitors. The preclinical development program has demonstrated that NVP018 has unique characteristics that make it a promising anti-viral drug candidate and the new research results underscore NVP018’s clinical potential for treating chronic hepatitis B infection.
“The results of the preclinical tests of NVP018, conducted by bodies including the prestigious Scripps Research Institute are very promising, with convincing data that cyclophilin inhibitors have a positive effect to hepatitis B virus infection. NVP018 is currently in a preclinical program, to be administered on patients at a later stage in clinical trials,” commented Jan Nilsson, COO of NeuroVive.
Current status of NeuroVive products
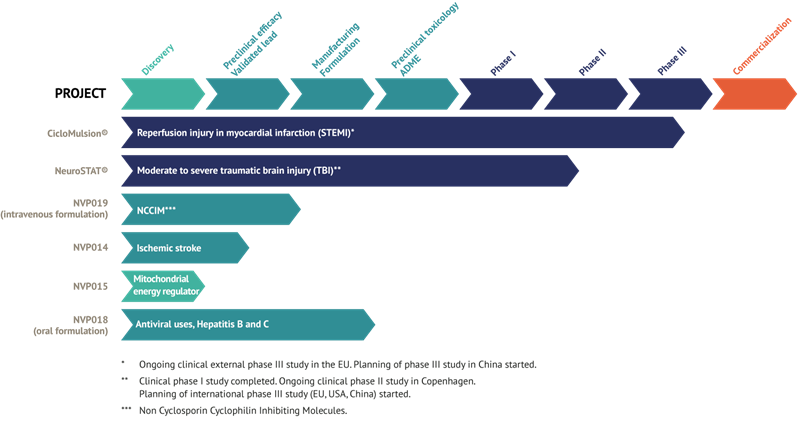
CicloMulsion®
NeuroVive’s product CicloMulsion® is the first cyclophilin inhibitor developed for treating reperfusion injury. The product’s potential for treatment of myocardial infarction is currently being evaluated in a clinical phase III study. The final participant of a total of 972 patients was enrolled on 16 February 2014. The results of this study are scheduled for presentation in 2015 after a one-year follow-up is completed on all patients and study data has been compiled.
NeuroSTAT®
NeuroVive is developing NeuroSTAT® for treating patients with severe traumatic brain injury (TBI). NeuroSTAT® is currently being evaluated in a clinical phase IIa study at Rigshospitalet, the Copenhagen teaching hospital. The study is focusing on safety and pharmacokinetics, and 5 of a planned 20 patients have been enrolled. The design and planning work for a phase III study has commenced. The company has obtained orphan drug designation for NeuroSTAT® for moderate to severe traumatic brain injury in the US and EU. Orphan drug designation confers market exclusivity for 7 years in the US and 10 years in the EU from the date when the company secures marketing authorization.
NVP018
The recently acquired cyclophilin inhibitors are part of the family of molecules called Sangamides, and based on a new and unique chemical platform of what are known as polyketides. NVP018 is NeuroVive’s primary drug candidate in the company’s new portfolio of potent cyclophilin inhibitors. It has undergone extensive preclinical development and has been developed for treating hepatitis B/C. This product demonstrates potent efficacy against virus replication and has a positive safety and pharmacokinetic profile. Cyclophilin inhibitors have the potential for broad-based application, and NeuroVive is currently evaluating NVP018’s potential for other viral indications.
Other products
More information on all products developed by NeuroVive is available at www.neurovive.se/index.php/en/research-development/our-products
About NeuroVive
NeuroVive Pharmaceutical AB (publ), a leading mitochondrial medicine company, is developing a portfolio of products to treat acute cardiovascular and neurological conditions through mitochondrial protection. These medical conditions are characterized by a pressing medical need and have no approved pharmaceutical treatment options at present. NeuroVive’s products CicloMulsion® (heart attack) and NeuroSTAT® (traumatic brain injury) are currently being evaluated in phase III and phase II studies, respectively. NeuroVive’s research programs also include products for the treatment of anti-viral indications (hepatitis B/C), brain cell injury in stroke patients, and drug candidates for cellular protection and treating mitochondria-related energy regulation diseases. NeuroVive’s shares are listed on NASDAQ OMX, Stockholm, Sweden.
For media and investor relations questions, please contact:
Ingmar Rentzhog, Laika Consulting, tel: +46 (0)46 275 62 21 or ir@neurovive.se
It is also possible to arrange an interview with NeuroVive’s CEO Mikael Brönnegård via the contact above.
NeuroVive Pharmaceutical AB (publ)
Medicon Village, SE-223 81 Lund, Sweden
Tel: +46 (0)46 275 62 20 (switchboard), Fax: + 46 (0)46 888 83 48, info@neurovive.se, www.neurovive.se
NeuroVive Pharmaceutical AB (publ) is required to publish the information in this news release under The Swedish Securities Market Act. The information was submitted for publication on 28 March 2014, at 8:30 a.m. CET.