“NeuroVive’s new pharmaceutical substances attracted considerable interest at the conference, and there is no existing treatment for diseases caused by mitochondrial energy production dysfunction. Overall, this validates our focus on this new drug class through animal testing, toxicology and clinical studies,” commented Jan Nilsson, NeuroVive’s COO.
“It’s incredible to move from an academic idea to tangible results in the way we’ve done with energy regulators. Our partnerships with the chemists at Selcia/Mitopharm Ltd and the Mitochondrial Medicine Unit at Lund University have been crucial to the effective development of these new pharmaceutical substances. NeuroVive researcher Johannes Ehinger was able to put our findings across very successfully in his presentation at the symposium,” commented Eskil Elmér, NeuroVive’s CSO.
NeuroVive’s energy regulators
In simplified terms, the mitochondria can be considered as the energy factory of cells. Diseases and medical conditions that affect mitochondrial function can cause heart and brain damage, and injure other organs with high energy requirements. The enzyme complex I is a central component in mitochondrial energy production. This first link in the respiratory chain is often affected in congenital dysfunction of energy production and in different medical conditions, and is sensitive to adverse events from pharmaceuticals. Succinic acid is a nutrient that is able to circumvent complex I in the respiratory chain, restoring energy production. However, succinic acid is normally unable to transit the cell wall independently. Alongside Selcia/Mitopharm Ltd., researchers linked to NeuroVive have resolved this problem by chemically altering succinic acid in various ways, with the result that the compound is able to transit the cell wall and then be freed up and utilized in mitochondrial energy production. The efficacy and potential adverse events from the new pharmaceutical substances must be examined in animal studies before they can be trialed on humans. One of the substances is now in a formulation suitable for animal studies and the first major validation study will be completed in 2014.
UMDF’s annual symposium
The United Mitochondrial Disease Foundation (UMDF) is an American non-profit organization that supports research and training relating to diagnostics and treatment of mitochondrial disease. UMDF was founded in 1996 and has raised close to USD 11 m for research into mitochondrial medicine. UMDF’s annual symposium brings together close to 600 participants every year, including leading researchers and medical professionals in mitochondrial medicine. The symposium is both a scientific conference and a meeting point for patients and patient organizations. Mitochondrial Medicine 2014 was held in Pittsburgh on 4-7 June.
NeuroVive made three presentations at the conference, presenting scientific data on the effect of energy regulators on human cells, the effect on adverse events from diabetes medication Metformin and the animal model developed for validation of the efficacy of the new pharmaceutical substances in mitochondrial disease. There’s more information on UMDF and the conference at www.umdf.org.
About NeuroVive Pharmaceutical
NeuroVive Pharmaceutical AB (publ), a leading mitochondrial medicine company, is developing a portfolio of products to treat acute cardiovascular and neurological conditions through mitochondrial protection. These medical conditions are characterized by a pressing medical need and have no approved pharmaceutical treatment options at present. NeuroVive’s products CicloMulsion® (heart attack) and NeuroSTAT® (traumatic brain injury) are currently being evaluated in phase III and phase II studies, respectively. NeuroVive’s research programs also include products for the treatment of anti-viral indications (Hepatitis B/C), brain cell injury in stroke patients, and drug candidates for cellular protection and treating mitochondria-related energy regulation diseases. NeuroVive’s shares are listed on NASDAQ OMX, Stockholm, Sweden.
Current status of NeuroVive’s products
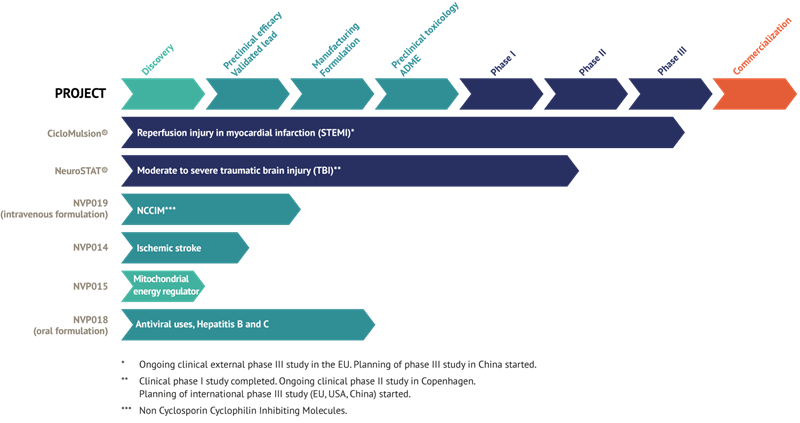
CicloMulsion®
NeuroVive’s product CicloMulsion® is the first cyclophilin inhibitor developed for the treatment of reperfusion injury. The product’s potential in the treatment of myocardial infarction is currently being evaluated in a clinical phase III study. The last of a total of 972 patients was enrolled on 16 February 2014. The results of the study are due to be announced in 2015 following the completion of the one-year follow-up of all patients and the presentation of the study data.
NeuroSTAT®
NeuroVive is developing NeuroSTAT® for the treatment of patients with severe traumatic brain injury. NeuroSTAT® is currently being evaluated in a clinical phase IIa study at Copenhagen University Hospital. The study focuses on safety and pharmacokinetics, and 5 of 20 planned patients have been enrolled so far. A phase III study is currently being planned and designed. NeuroVive has secured orphan drug designation for NeuroSTAT® for moderate and severe traumatic brain injury in the US and EU, which implies market exclusivity for seven years in the US and ten years in the EU, from the date NeuroVive obtains market authorization.
NVP018
The recently acquired cyclophilin inhibitors are part of a family of molecules known as Sangamides, and are based on a new and unique chemistry platform of what are termed polyketides. NVP018 is NeuroVive’s primary drug candidate in the company’s new portfolio of potent cyclophilin inhibitors. It has undergone extensive pre-clinical development and has been developed for the treatment of Hepatitis B/C. The product has demonstrated high potency against virus replication and has a positive safety and pharmacokinetic profile. Cyclophilin inhibitors have broad-based applications and NeuroVive is currently evaluating NVP018’s potential for other anti-viral indications.
NVP019
NVP019 is based on the same active substance as NVP018 and is being developed as the next generation cyclophilin inhibitor for acute heart and nerve cell damage, but also for other acute heart conditions and acute conditions where general protection of the vital organs is central to the progression of the disease. An intravenous preparation form will be evaluated for this purpose in collaboration with external parties such as Hospices Civils de Lyon within the framework of the OPeRa program.
Other products
More information about all products developed by NeuroVive can be found at http://www.neurovive.se/index.php/en/research-development/research-overview
For Investor Relations and media questions, please contact:
Ingmar Rentzhog, Laika Consulting, Tel: +46 (0)46 275 62 21 or ir@neurovive.se
It is also possible to arrange an interview with NeuroVive’s CEO Mikael Brönnegård at the above contact.
NeuroVive Pharmaceutical AB (publ)
Medicon Village, SE-223 81 Lund, Sweden
Tel: +46 (0)46 275 62 20 (switchboard), Fax: +46 (0)46 888 83 48
info@neurovive.se, www.neurovive.se
NeuroVive Pharmaceutical AB (publ) is required to publish the information in this news release under The Swedish Securities Market Act. The information was submitted for publication on 10 June 2014, at 08.30 a.m. CET.