The ongoing CHIC study (Copenhagen Head Injury Ciclosporin Study) is an open-label, non-comparative clinical Phase IIa study enrolling a total of 20 patients divided into two different dosage groups, where NeuroVive’s drug candidate NeuroSTAT® is being evaluated for the treatment of patients with traumatic brain injury. The study is being conducted at the Department of Neurosurgery at Rigshospitalet, University of Copenhagen, with MD. Jesper Kelsen as Principal Investigator.
The study’s first dosage group of 10 patients has now been treated with NeuroSTAT® at the lower dose, and an interim analysis has been completed by an independent safety committee in order to evaluate the treatment’s safety profile. The analysis includes an evaluation of blood concentrations of cyclosporin A (the active substance in NeuroSTAT®) and changes in intracranial pressure and blood samples collected to analyze possible organ injury. According to the analysis, the low-dose treatment is judged to be safe and the study will now continue as planned with the higher dosage group including 10 additional patients.
“We’ve now obtained important safety data on what we’ve designated to be the lower dose of NeuroSTAT® for treating patients with traumatic brain injury. We can now move on to include patients that will be treated with a higher dose. This means that the study has reached an important milestone in the clinical trial program of NeuroSTAT®,” commented NeuroVive’s CEO Mikael Brönnegård.
More information about the study
The primary objective of the CHIC study is to evaluate NeuroSTAT®’s safety and pharmacokinetics in blood and cerebrospinal fluid of patients with severe traumatic brain injury on the basis of two different dosage levels. Secondary explorative measures will be completed to study NeuroSTAT®’s efficacy at the mitochondrial level and to study how different biochemical processes are affected by NeuroSTAT® following traumatic brain injury. In addition to interim analysis, the safety profile of the treatment is evaluated continuously. More information about the study has been published in the public database ClinicalTrials.gov at: https://clinicaltrials.gov/ct2/show/NCT01825044
Current status of NeuroVive’s projects and drug candidates
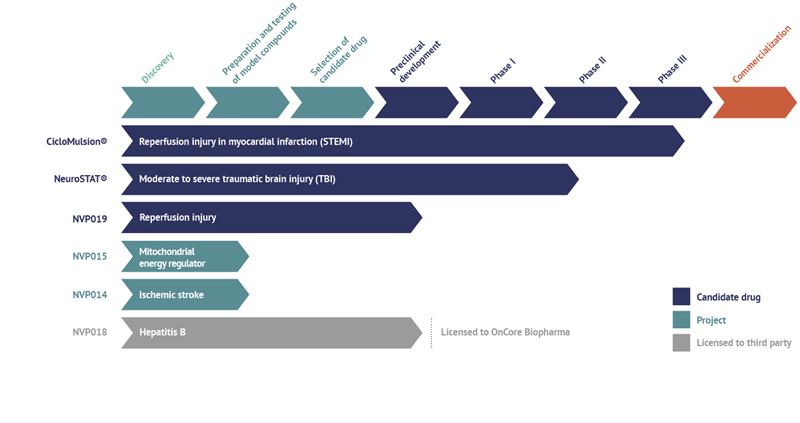
CicloMulsion®
NeuroVive’s product CicloMulsion® is the first cyclophilin inhibitor developed for the treatment of reperfusion injury. The product’s potential in the treatment of myocardial infarction is currently being evaluated in a clinical phase III study. The last of a total of 972 patients was enrolled on 16 February 2014. The results of the study are due to be announced in the third quarter 2015 following the completion of the one-year follow-up of all patients and the analysis of the study data. CicloMulsion® will also be evaluated in a number of clinical phase II studies for the treatment of other acute cardiac and kidney injury within the framework of the collaboration with Hospices Civils de Lyon and Skånes University Hospital in Lund, Sweden.
NeuroSTAT®
NeuroVive is developing NeuroSTAT® for the treatment of patients with moderate or severe traumatic brain injury. NeuroSTAT® is currently being evaluated in a clinical phase IIa study at Copenhagen University Hospital. The study focuses on safety and pharmacokinetics, and 10 of 20 planned patients have been enrolled so far. A phase III study is currently being planned and designed. NeuroVive has secured orphan drug designation for NeuroSTAT® for moderate and severe traumatic brain injury in the US and EU, which implies market exclusivity for seven years in the US and ten years in the EU, from the date NeuroVive obtains market authorization.
NVP019
NVP019 is NeuroVive’s primary drug candidate in the company’s new portfolio of potent cyclophilin inhibitors belonging to a family of molecules known as Sangamides based on a new and unique polyketide engineering technology. NVP019 is being developed as the next generation cyclophilin inhibitor for the treatment of reperfusion injury in myocardial infarct, but also for other acute conditions where general protection of vital organs is central to the progression of the disease. An intravenous formulation will be evaluated for this purpose in collaboration with external parties such as Hospices Civils de Lyon within the framework of the OPeRa program.
NVP018
NVP018 is an oral formulation based on the same active substance as NVP019. It has been developed for treatment of Hepatitis B and was outlicensed to OnCore Biopharma, Inc. (www.oncorebiopharma.com) in September 2014. OnCore Biopharma has designated the drug candidate OCB030.
Other products
More information about all products developed by NeuroVive can be found at http://www.neurovive.se/index.php/en/research-development/research-overview
About NeuroVive
NeuroVive Pharmaceutical AB (publ), the mitochondrial medicine company, is developing a portfolio of products to treat acute cardiovascular and neurological conditions through mitochondrial protection. These medical conditions are characterized by a pressing medical need and have no approved pharmaceutical treatment options at present. NeuroVive’s products CicloMulsion® (myocardial infarct) and NeuroSTAT® (traumatic brain injury) are currently being evaluated in phase III and phase II studies, respectively. NeuroVive’s research programs also include development of treatments against brain injury in stroke patients, and drug substances for cellular protection and treatment of mitochondrial disorders causing energy deficiency. NeuroVive’s shares are listed on NASDAQ OMX, Stockholm, Sweden.
For Investor Relations and media questions, please contact:
Ingmar Rentzhog, Laika Consulting, Tel: +46 (0)46 275 62 21 or ir@neurovive.se
It is also possible to arrange an interview with NeuroVive’s CEO Mikael Brönnegård or COO Jan Nilsson at the above contact.
NeuroVive Pharmaceutical AB (publ)
Medicon Village, SE-223 81 Lund, Sweden, Tel: +46 (0)46 275 62 20 (switchboard), Fax: +46 (0)46 888 83 48
info@neurovive.se, www.neurovive.se
NeuroVive Pharmaceutical AB (publ) is required to publish the information in this news release under The Swedish Securities Market Act. The information was submitted for publication on 21 April 2015, at 9.45 a.m. CET.