NeuroVive’s product CicloMulsion® is already being evaluated in clinical studies focusing on protecting the heart and brain in connection with acute injury (known as NeuroSTAT® for brain protection). Drugs that protect the mitochondria also have significant potential to protect high-energy organs like the kidneys during surgery. Two clinical studies have shown that the active substance is safe for heart surgery and provides protection against diffuse injury*. This has led Skåne University Hospital to initiate a clinical study to evaluate the potential for protecting the kidneys using CicloMulsion® in cardiac surgery.
The study is known as CiPRICS (Ciclosporin to Protect Renal function In Cardiac Surgery) and is a double-blind, randomized and placebo-controlled clinical phase II study to include a total of 150 patients. The patients will be treated with CicloMulsion® in connection with coronary artery bypass surgery at the Clinic for Cardiothoracic Surgery at Skåne University Hospital in Lund, Sweden. The study is investigator-initiated and is conducted with support from NeuroVive.
“Acute kidney injury is a serious problem that has received increasing attention in recent years. We know that patients who suffer from a transient reduction in renal function in connection with heart surgery have a poorer prognosis, and there are currently no preventive treatments available. Cyclosporin-A, the active substance in CicloMulsion®, is very promising in this context. What’s unique about our study is that we’ll focus specifically on patients with the highest risk of kidney injury and will monitor renal function very closely in the days following surgery. We’re very pleased to be collaborating with NeuroVive, which enables us to conduct this important study” commented Henrik Bjursten, Associate Professor and Senior Consultant at Skåne University Hospital and the researcher responsible for the study.
“The CiPRICS study meets a pressing medical need and is also another step towards evaluating new indications for our cyclophilin inhibitor CicloMulsion® and the forthcoming product NVP019. This means that we take a very positive view of this collaboration with Skåne University Hospital. The prevention of acute kidney injury during heart surgery is a promising indication, as we’d be able to pre-treat patients and our drug candidates would be present throughout the period organs are exposed to altered blood supply” commented NeuroVive’s CEO Mikael Brönnegård.
About mitochondrial protection of the kidneys during heart surgery
During cardiac surgery, the heart is usually stopped during surgery to enable procedures such as heart valve repair or coronary artery bypass grafts. A heart-lung machine is used to oxygenate and pump blood to the body while the heart is stopped. This means that heart surgery causes stress to the body as a whole, not just the heart, in the form of altered blood flow, and there is a pressing need to protect high-energy organs such as the kidneys from serious damage. This applies particularly to patients where renal function is already compromised prior to surgery and where the risk of complications is considered the greatest. The treatment is potentially suitable for all patients undergoing heart surgery using a heart-lung machine. In 2010, some 700,000 such surgeries were completed in the US, and NeuroVive estimates that the figure for Europe is similar. In other words, there is extensive medical need and market potential for the treatment.
*). Hausenloy DJ et al. Heart 2014;100:544–9 & Chiari P et al. Anesthesiology. 2014;121:232-8
Current status of NeuroVive’s projects and drug candidates
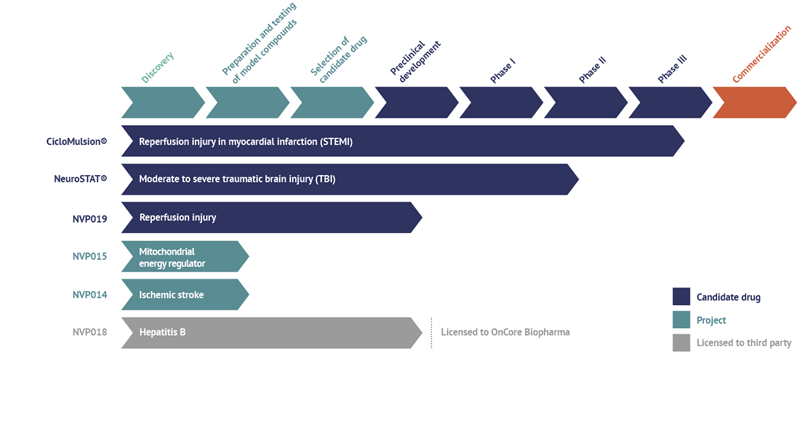
CicloMulsion®
NeuroVive’s product CicloMulsion® is the first cyclophilin inhibitor developed for the treatment of reperfusion injury. The product’s potential in the treatment of myocardial infarction is currently being evaluated in a clinical phase III study. The last of a total of 972 patients was enrolled on 16 February 2014. The results of the study are due to be announced in 2015 following the completion of the one-year follow-up of all patients and the analysis of the study data. CicloMulsion will also be evaluated in a number of clinical phase II studies for the treatment of other acute heart and kidney injury within the framework of the collaboration with Hospices Civils de Lyon and Skåne University Hospital in Lund, Sweden.
NeuroSTAT®
NeuroVive is developing NeuroSTAT® for the treatment of patients with moderate or severe traumatic brain injury. NeuroSTAT® is currently being evaluated in a clinical phase IIa study at Copenhagen University Hospital. The study focuses on safety and pharmacokinetics, and 8 of 20 planned patients have been enrolled so far. A phase III study is currently being planned and designed. NeuroVive has secured orphan drug designation for NeuroSTAT® for moderate and severe traumatic brain injury in the US and EU, which implies market exclusivity for seven years in the US and ten years in the EU, from the date NeuroVive obtains market authorization.
NVP019
NVP019 is NeuroVive’s primary drug candidate in the company’s new portfolio of potent cyclophilin inhibitors belonging to a family of molecules known as Sangamides based on a new and unique polyketide engineering technology. NVP019s being developed as the next generation cyclophilin inhibitor for reperfusion injury in heart attack, but also for other acute conditions where general protection of vital organs is central to counteracting the progression of the disease. An intravenous formulation will be evaluated for this purpose in collaboration with external parties such as Hospices Civils de Lyon within the framework of the OPeRa program.
NVP018
NVP018 is an oral formulation based on the same active substance as NVP019. It has been developed for treatment of Hepatitis B and was outlicensed to OnCore Biopharma (www.oncorebiopharma.com) in September 2014.
Other products
More information about all products developed by NeuroVive can be found at http://www.neurovive.se/index.php/en/research-development/research-overview
About NeuroVive Pharmaceutical
NeuroVive Pharmaceutical AB (publ), a leading mitochondrial medicine company, is developing a portfolio of products to treat acute cardiovascular and neurological conditions through mitochondrial protection. These medical conditions are characterized by a pressing medical need and have no approved pharmaceutical treatment options at present. NeuroVive’s products CicloMulsion® (heart attack) and NeuroSTAT® (traumatic brain injury) are currently being evaluated in phase III and phase II studies, respectively. NeuroVive’s research programs also include products for the treatment of brain cell injury in stroke patients, and drug candidates for cellular protection and treating mitochondria-related energy regulation diseases. NeuroVive’s shares are listed on NASDAQ OMX, Stockholm, Sweden.
For Investor Relations and media questions, please contact:
Ingmar Rentzhog, Laika Consulting, Tel: +46 (0)46 275 62 21 or ir@neurovive.se
It is also possible to arrange an interview with NeuroVive’s CEO Mikael Brönnegård or COO Jan Nilsson at the above contact.
NeuroVive Pharmaceutical AB (publ)
Medicon Village, SE-223 81 Lund, Sweden
Tel: +46 (0)46 275 62 20 (switchboard), Fax: +46 (0)46 888 83 48
info@neurovive.se, www.neurovive.se
NeuroVive Pharmaceutical AB (publ) is required to publish the information in this news release under The Swedish Securities Market Act. The information was submitted for publication on 2 December 2014, at 8.30 a.m. CET.